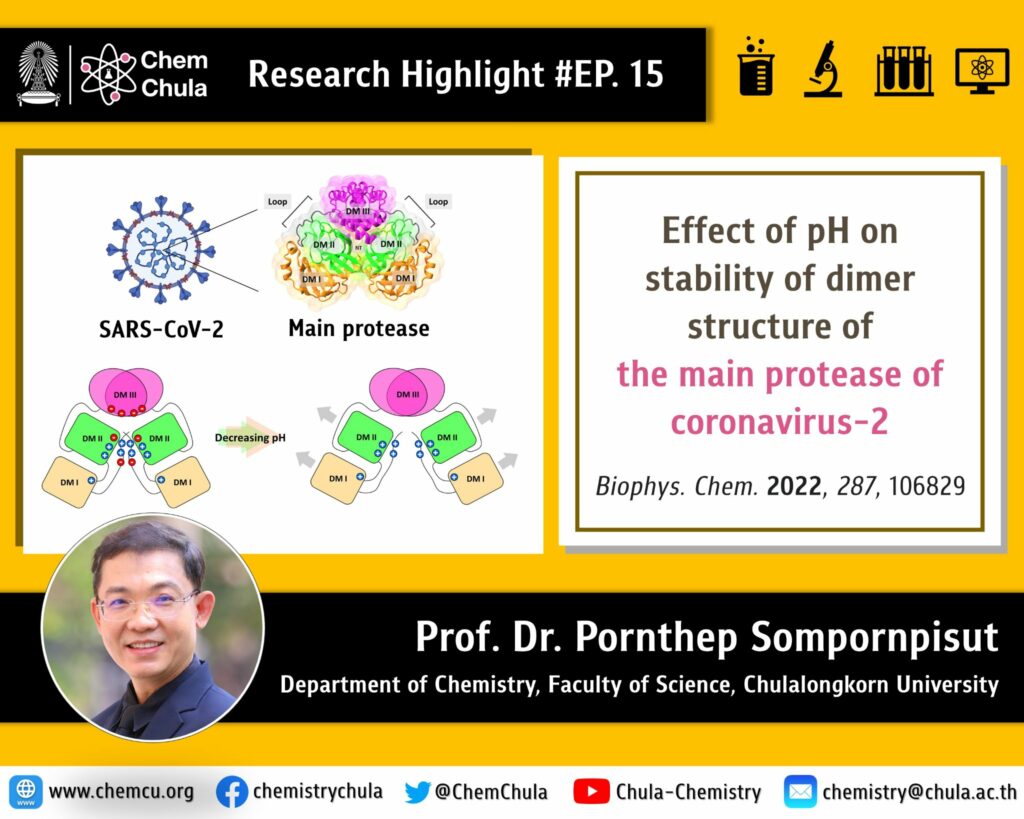
Understanding the structure and function of proteins in the coronavirus (COVID-19) is crucial to the drug development. The 3C-like main protease from SARS-CoV-2 is a key protein that can potentially be used as a drug target molecule. This protein plays a crucial role in the viral proliferation and replication. There have been shown that protein activity decreases as pH changes from neutral to acidic conditions. Prof. Dr. Pornthep’s research group studied the structure, dynamics and interactions of proteins through molecular dynamics simulations. The results showed that some regions of the protein exhibit clear structural differences between the two pH conditions. In acidic conditions, the structure of proteins appears to be less stable with respect to neutral pH. This study can identify amino acids that loose intramolecular hydrogen bonds and their interactions, leading to a dimer dissociation of the protein.
- Pornthep Sompornpisut
- R.B. Pandey
- Panisak Boonamnaj