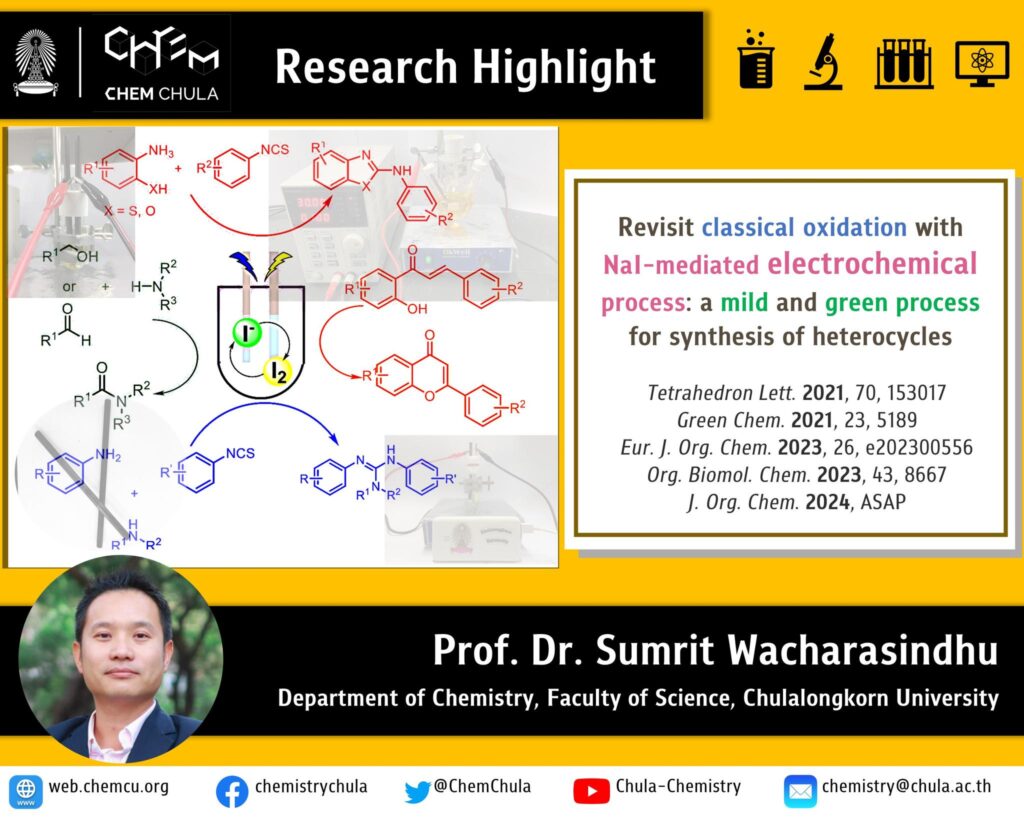
Simple oxidation reaction is one of the most common reactions in industry and academic laboratory. However, it is one of the most polluting and costly. One of the well-known oxidizing agents for constructing C-N bond is iodine. Although the reaction is efficient, they suffered from the use of stoichiometric amounts reagents. Over the past few decades, electrosynthesis has emerged as a sustainable and environmentally friendly method for molecular transformation. Among them, the utilization of iodine-derived agents generated in situ via the electrochemical method has gained much attention for several reasons: 1) the potent oxidizing capability of these agents, 2) recyclability, enabling their use in catalytic amount, and 3) dual functionality as both electrolyte and mediator of precursor iodide salts. For the past few years, Prof. Sumrit Wacharasindhu’s research group has utilized this emerging electrochemical method to prepare various heterocycle such as amides, 2-aminobenzoxazoles, flavone, guanidines and 2-aminobenzimidazoles.
- Thao Nguyen Thanh Huynh
- Khuyen Thu Nguyen
- Mongkol Sukwattanasinitt
- Sumrit Wacharasindhu