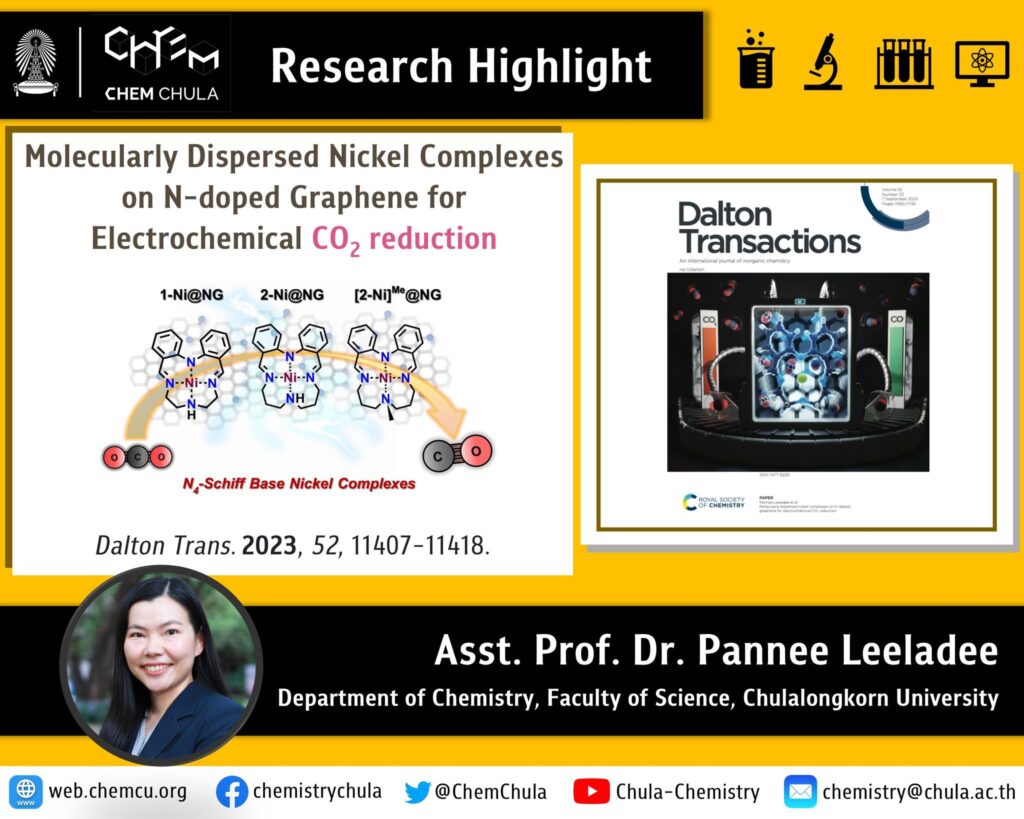
The burning of fossil fuels, deforestation, and industrial processes are releasing increasing amounts of carbon dioxide (CO2) into the atmosphere, leading to serious environmental problems such as global warming. Electrochemical CO2 conversion has emerged as a promising strategy for harnessing CO2 as a chemical feedstock to synthesize value-added chemicals (e.g., carbon monoxide, formic acid, methanol, methane) while concurrently mitigating these environmental challenges. The research group of Asst. Prof. Pannee Leeladee has investigated the electrochemical conversion of CO2 employing catalysts made of nickel complexes dispersed on N-doped graphene (Ni@NG). In an aqueous medium, utilizing sodium bicarbonate (NaHCO3) as the electrolyte, Ni@NG catalysts exhibited satisfactory CO2-to-CO reduction performance with a faradaic efficiency ranging from 60-80% at an overpotential of 0.56 V versus the Reversible Hydrogen Electrode (RHE).
- Methasit Juthathan
- Teera Chantarojsiri
- Kittipong Chainok
- Teera Butburee
- Patchanita Thamyongkit
- Thawatchai Tuntulani
- Pannee Leeladee