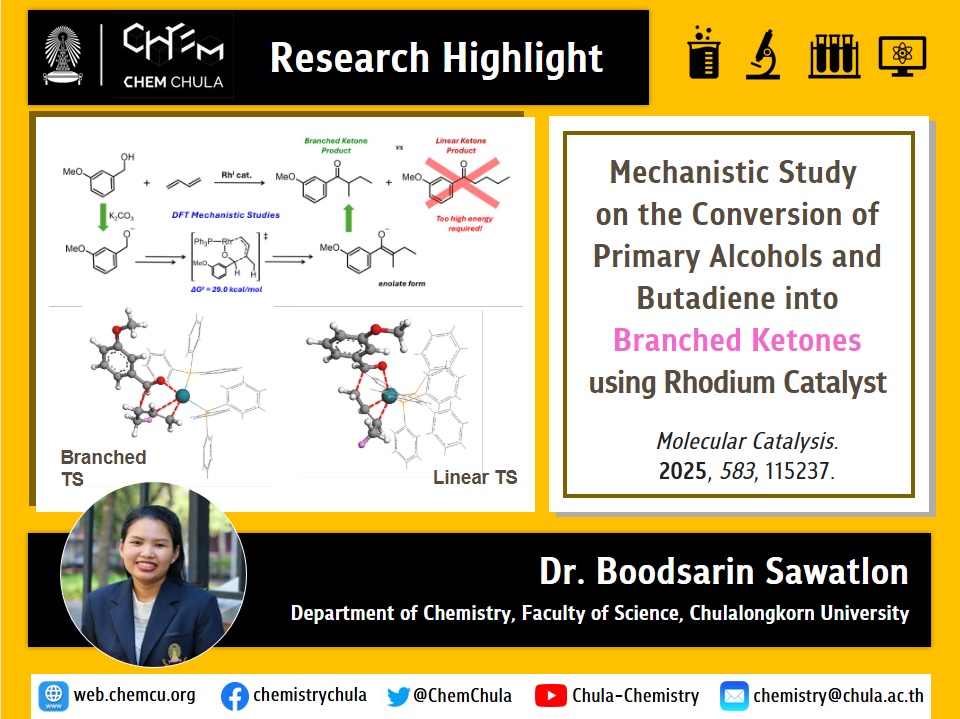
This work investigates the mechanism of a Rh(I)-catalyzed transformation of primary alcohols and butadiene into saturated branched ketones, without premetalated intermediates or external hydrogen sources. DFT calculations reveal a four-step pathway involving alcohol oxidation, butadiene hydrogenation, carbonyl addition, and intramolecular hydrogen transfer. The rate-determining step is the sterically hindered carbonyl addition, which also explains why only the branched product is observed. The results support a hydrogen autotransfer mechanism consistent with experimental labeling studies.
- Chaiyaporn Lakmuang
- Natcha Prommoon
- Natnicha Namwong
- Vudhichai Parasuk
- Boodsarin Sawatlon