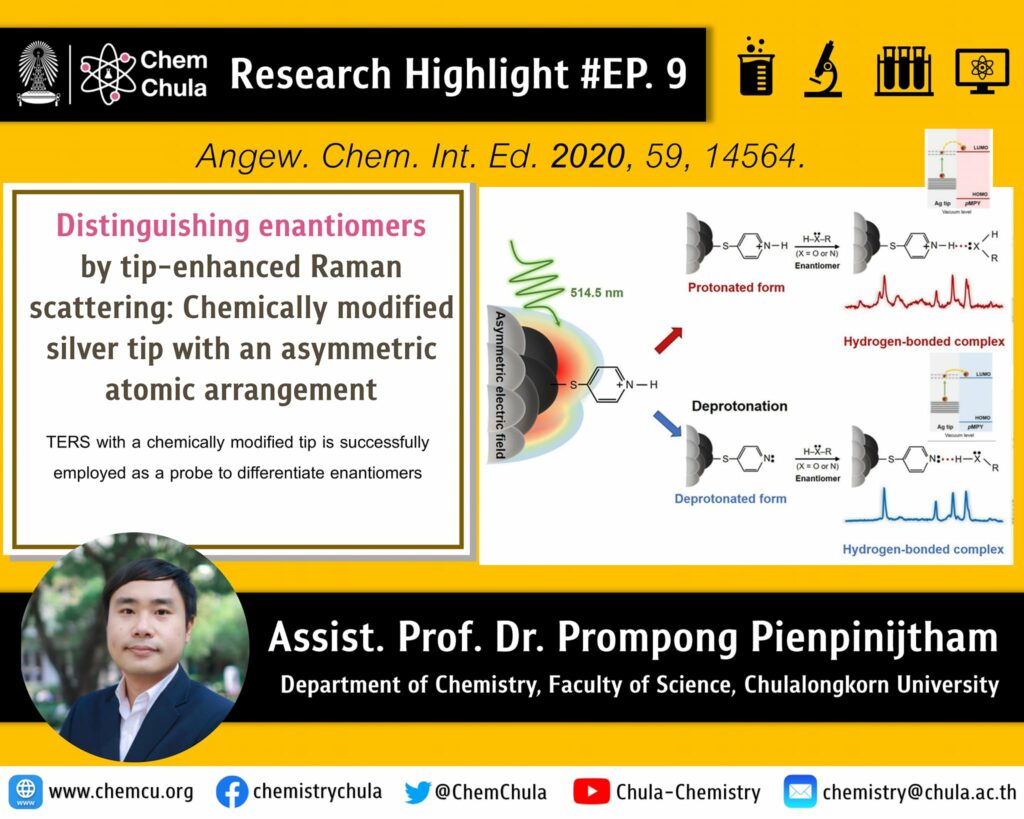
Discrimination between enantiomers is achieved by tip-enhanced Raman scattering using a silver tip that is chemically modified by an achiral para-mercaptopyridine (pMPY) probe molecule. Differences in the relative intensities of the pMPY spectra were monitored for three pairs of enantiomers containing hydroxy (–OH) and/or amino (–NH2) groups. The N: or N+–H functionality of the pMPY-modified tip participates in hydrogen-bond interactions with a particular molecular orientation of each chiral isomer. The asymmetric arrangement of silver atoms at the apex of the tip induces an asymmetric electric field, which causes the tip to become a chiral center. Differences in the charge-transfer states of the metal-achiral probe system in conjunction with the asymmetric electric field produce different enhancements in the Raman signals of the two enantiomers. The near-field effect of the asymmetric electric field, which depends on the number of analyte functional groups capable of hydrogen-bond formation, improves the degree of discrimination.
- Prompong Pienpinijtham
- Thanyada Sukmanee
- Kanet Wongravee
- Sanong Ekgasit
- Tamitake Itoh
- Yukihiro Ozaki