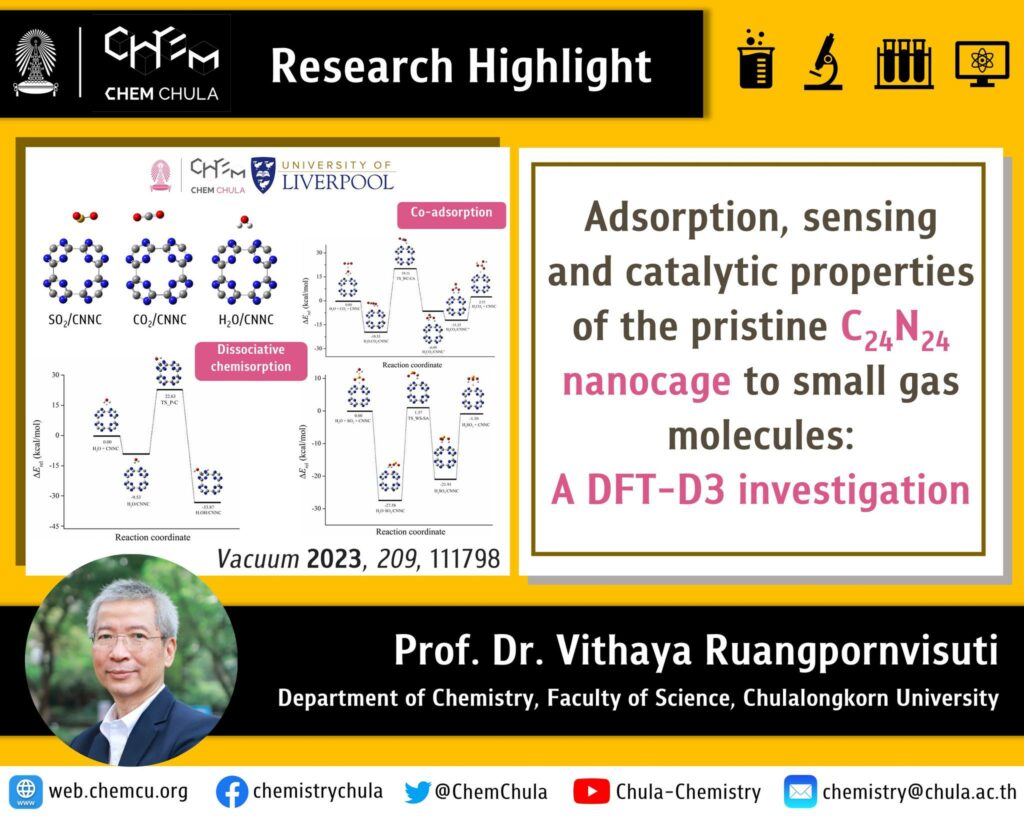
The carbon nitride nanocage (CNNC), which is new nanomaterial, has potential to adsorb a range of simple gasses onto either nitrogen site or carbon site. Adsorption of different gases on CNNC will have different adsorption energies. The high adsorption energies indicate the high stability of complexes that exhibit the potential to be gas storage materials. The gas adsorption can result in electrical conductivity. If the gas adsorption on surface affects to electrical conductivity significantly and has a low recovery time, this nanocage has the potential to be gas sensing material.
The research group of Prof. Dr. Vithaya Ruangpornvisuti collaborated with student internship from the Department of Chemistry, University of Liverpool, UK, to study adsorption and sensing properties on pristine C24N24 nanocage (CNNC) to small gas molecules using density functional theory (DFT).
The results showed that the CNNC can adsorb small gas molecules and was suggested to be a gas storage material. On the CNNC surface, co-adsorption of H2O and SO2, which was assumed as Langmuir-Hinshelwood mechanism, and co-adsorption of H2O and CO2, which was assumed as Langmuir-Rideal mechanism, were found. Additionally, H2O physisorption on CNNC surface to form dissociative chemisorption was found. The small gas molecules adsorption on surface of nanomaterials is essential for research and development in catalyst and energy storage.
- Vithaya Ruangpornvisuti
- Thomas Condon-Baxendale
- Nontawat Ploysongsri
- Monrada Petchmark